Does Color Change Indicate A Chemical Reaction
Chemical Alter vs. Physical Change
- Page ID
- 346
The difference between a physical reaction and a chemic reaction is composition. In a chemical reaction, there is a change in the composition of the substances in question; in a physical change in that location is a difference in the advent, scent, or unproblematic display of a sample of thing without a alter in composition. Although we call them physical "reactions," no reaction is really occurring. In order for a reaction to accept place, there must be a change in the elemental composition of the substance in question. Thus, we shall simply refer to physical "reactions" as concrete changes from now on.
Introduction
Concrete changes are limited to changes that result in a difference in display without changing the composition. Some common changes (merely non express to) are:
- Texture
- Color
- Temperature
- Shape
- Change of Country (Boiling Point and Melting Point are significant factors in determining this change.)
Physical backdrop include many other aspects of a substance. The post-obit are (just not limited to) concrete backdrop.
- Luster
- Malleability
- Ability to exist drawn into a thin wire
- Density
- Viscosity
- Solubility
- Mass
- Volume
Any change in these physical properties is referred to every bit a physical change. For further information, please refer to Backdrop of Matter.
Chemical changes, on the other hand, are quite different. A chemical modify occurs when the substance's limerick is inverse. When bonds are broken and new ones are formed a chemical change occurs. The post-obit are indicators of chemical changes:
- Change in Temperature
- Change in Color
- Noticeable Aroma (after reaction has begun)
- Germination of a Precipitate
- Germination of Bubbles
Notation: When 2 or more reactants are mixed and a change in temperature, color, etc. is noticed, a chemical reaction is probably occurring. These are not definite indicators; a chemical reaction may not exist occurring. A change in colour is not always a chemical change. If ane were to change the color of a substance in a non-chemical reaction scenario, such as painting a motorcar, the change is concrete and not chemical. This is considering the composition of the machine has not changed. Keep with caution.
Mutual Physical Changes
Texture
The texture of a substance can differ with a physical modify. For example, if a piece of wood was sanded, waxed, and polished, it would have a very different texture than it initially had every bit a rough piece of wood.

(left) Rough plank boardwalk, Quebec Urban center, Canada (right) Finished mountain ash floor. (CC By-SA four.0; WikiPedant and CC By-SA 2.5; MarkAnthonyBoyle, respectively).
Equally y'all can see, the texture of the finished forest is much smoother than the initial grainy wood.
Color
The changing of color of a substance is not necessarily an indicator of a chemic alter. For example, changing the color of a metallic does non change its physical properties. However, in a chemical reaction, a color modify is ordinarily an indicator that a reaction is occurring. Painting the metal automobile does not changing the limerick of the metal substance.
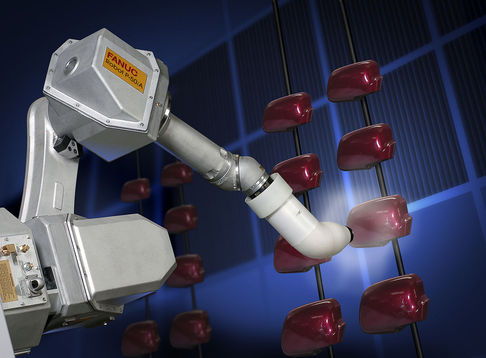
Robotic arm applying paint on machine parts. Image apply with permission (CC Past-SA 4.0l RoboGuru).
Temperature
Although we cannot come across temperature change, unless if a alter of state is occurring, it is a physical change.

Hot metalwork. (CC BY-SA-NC two.0; flagstaffotos.com.au)
I cannot see the pan physically changing shape, colour, texture, or any of the other concrete backdrop. However, if one were to bear upon the pan, it would be incredibly hot and could cause a burn down. Sitting idle in a closet, this pan would be cold. One cannot assess this modify only through visual exposure; the use of a thermometer or other instrument is necessary.
Shape
The shape of an object can be changed and the object will nonetheless remain true to its chemical composition. For case, if one were to fold coin, equally shown by the figure below, the money is still chemically the same.
Origami Money

Modify of State
The modify of state is besides a concrete change. In this scenario, 1 tin can discover a number of concrete properties irresolute, such as viscosity and shape. Every bit ice turns into water, it does not retain a solid shape and at present becomes a sticky fluid. The physical "reaction" for the alter of ice into liquid water is:
\[H_2O_{(southward)} \rightarrow H_2O_{(fifty)}\]

The following are the changes of state:
| Solid → Liquid | Melting |
| Liquid → Gas | Vaporization |
| Liquid → Solid | Freezing |
| Gas → Liquid | Condensation |
| Solid → Gas | Sublimation |
- If rut is added to a substance, such equally in melting, vaporization, and sublimation, the process is endothermic. In this instance, estrus is increasing the speed of the molecules causing them movement faster.
- If heat is removed from a substance, such as in freezing and condensation, then process is exothermic. In this instance, estrus is decreasing the speed of the molecules causing them motility slower.
Physical Properties
Luster
The luster of an element is divers equally the way it reacts to light. Luster is a quality of a metal. Almost all of the metals, transition metals, and metalloids are lustrous. The non-metals and gases are non lustrous. For case, oxygen and bromine are non lustrous. Shown below is are lustrous paper clips.
Lustrous Paperclips

Malleability
Malleability is also a quality of metals. Metals are said to be malleable. This means that the metals tin deform under an amount of stress. For example, if you can striking a metallic with a mallet and information technology deforms, it is malleable. Also, a paperclip can exist shaped with bare hands.
Bent Paperclip

The prototype shows the malleability of a certain metallic equally stress is applied to it.
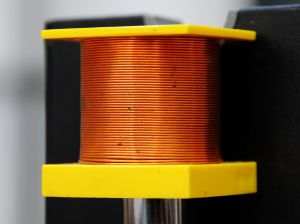
Ability to exist drawn into a sparse wire
In materials science, this property is called ductility. For example, raw copper can be obtained and it tin be purified and wrapped into a cord. Once again, this belongings is characteristic of mainly metals, nonmetals practise not possess this quality.
Copper Wire
Density
The density of an object is its mass divided by its volume (d=m/v). A substance will have a higher density if information technology has more than mass in a fixed corporeality of book. For instance, take a ball of metal, roughly the size of a baseball game, compressed from raw metal. Compare this to a baseball made of paper. The baseball made of metal has a much greater weight to it in the aforementioned amount of volume. Therefore the baseball made out of metal has a much higher density. The density of an object will also determine whether it will sink or float in a particular chemical. Water for instance has a density of 1g/cm3. Any substance with a density lower than that volition float, while any substance with a density higher up that volition sink.
Oil Sinking in a Glass of Water

Viscosity
Viscosity is defined to be the resistance to deformation of a particular chemical substance when a forcefulness is applied to information technology. In the example beneath, i can meet 2 cubes falling into 2 different exam tubes. The upper substance shows a trigger-happy reaction to the dropping of the cube. The lower substance simply engulfs it slowly without much reaction. The upper substance has a lower viscosity relative to the lower substance, which has very high viscosity. One may even think of viscosity in terms of thickness. The substance with more thickness has college viscosity than a substance that is accounted "thin." Water has a lower viscosity than love or magma, which take relatively high viscosities.
Viscosity of Fluids

Common Chemical Changes
The follow are all indicators of chemical reactions. For further information on chemic reactions, please refer to Chemical Reactions.
Change in Temperature
A change in temperature is characteristic of a chemical alter. During an experiment, ane could dip a thermometer into a beaker or Erlenmeyer Flask to verify a temperature change. If temperature increases, as it does in most reactions, a chemical alter is likely to be occurring. This is different from the physical temperature alter. During a physical temperature alter, one substance, such as water is being heated. However, in this instance, one compound is mixed in with another, and these reactants produce a product. When the reactants are mixed, the temperature change acquired by the reaction is an indicator of a chemic modify.

Every bit an example of a exothermic reaction, if \(Fe_2O_3\) is mixed with Al and ignighted (often with burning Mg), then the thermite reaciton is initiated
\[Fe_2O_3 + 2Al \rightarrow 2Fe + Al_2O_3 + \text{Heat}\]
This reaction generates heat as a product and is (very) exothermic.
However, physical changes can exist exothermic or endothermic. The melting of an water ice cube, which is endothermic, is a modify in a concrete belongings and not composition. Thus, it is a physical alter.
Change in Color
A change in colour is also another characteristic of a chemical reaction taking place. For example, if one were to observe the rusting of metal over time, one would realized that the metal has changed color and turned orange. This modify in color is bear witness of a chemical reaction. Still, one must be careful; sometimes a alter in color is just the mixing of two colors, but no real modify in the composition of the substances in question.
Metallic Rusting

The reaction higher up is that of the rusting of iron.
\[4Fe + 3O_2 + 6H_2O \rightarrow 4Fe(OH)_3\]
Noticeable Odor
When two or more compounds or elements are mixed and a scent or odor is nowadays, a chemical reaction has taken place. For example, when an egg begins to smell, (a rotten egg) a chemical reaction has taken identify. This is the effect of a chemical decomposition.
Spoiled Egg

Formation of a Precipitate
The formation of a precipitate may be 1 of the most mutual signs of a chemic reaction taking place. A precipitate is defined to exist a solid that forms within of a solution or another solid. Precipitates should not be confused with suspensions, which are solutions that are homogeneous fluids with particles floating nearly in them. For example, when a soluble carbonate reacts with Barium, a Barium Carbonate precipitate can be observed.
Test Tube

Reaction:
\[Ba^{2+}_{(aq)} + CO^{2-}_{three\;(aq)} \rightarrow BaCO){3\;(s)}\]
For further information, please refer to Classification of Matter.
Formation of Bubbles
The germination of bubbles, or rather a gas, is another indicator of a chemical reaction taking place. When bubbling grade, a temperature change could also be taking place. Temperature alter and formation of bubbles often occur together. For case, in the following paradigm, one can come across a gas spewing. This is the formation of a gas.
Gas Formation

However, well-nigh reactions are much more subtle. For case, if the following reaction occurs, 1 may notice Carbon Dioxide bubbles forming. If there is enough Hydrochloric acid, bubbles are visible. If there isn't, i can't readily notice the alter:
\[Na_2CO_3 + 2HCl \rightarrow 2NaCl + H_2O + CO_2\]
References
- Chang, Raymond. Full general Chemical science: the Essential Concepts. Boston, MA: McGraw-Colina College Education, 2006. Impress.
- Chemistry for Dummies. For Dummies, 2008. Print.
- Petrucci, Ralph H. Full general Chemistry Principles and Modernistic Applications. Upper Saddle River, NJ: Pearson/Prentice Hall, 2007. Print.
Exterior Links
All images are courtesy of http://world wide web.sxc.hu, which provides royalty free images that are gratuitous to be copied without restrictions. The viscosity prototype is likewise free to be duplicated equally per permission of writer on Wikipedia.com.
Problems
ane. Which of the following is a chemic reaction?
- Freezing liquid Mercury
- Adding yellow to blueish to make green
- Cutting a piece of newspaper into 2 pieces
- Dropping a sliced orange into a vat of Sodium Hydroxide
- Filling a balloon with natural air
2. Which of the post-obit is a physical reaction?
- Shattering Glass with a baseball
- Corroding Metal
- Fireworks Exploding
- Lighting a friction match
- Blistering a cake
iii. Which of the following is a chemic reaction?
- Painting a wall blueish
- A bicycle rusting
- Ice cream melting
- Scratching a key across a desk
- Making a sand castle
4. Which of the following is a concrete reaction?
- Frying an egg
- Digesting carrots
- A Macbook falling out of a window
- Creating ATP in the human trunk
- Dropping a fizzy tablet into a glass of water
5. Write C for Chemical Reaction or P for Physical Reaction.
- Burning Leaves
- Cutting Diamonds
- Burdensome a pencil
- The salivary amylase enzyme that breaks down food in the mouth
- Salt mixing in with water
Answers
one. D
two. A
three. B
4. C
v. a) C
b) P
c) P
d) C
e) Neither. This is one of the gray areas of chemic change and physical change. Although the salt has dissociated into Sodium and Chloride ions, it is still common salt in h2o. Salt, initially is actually just a conglomerate of sodium and chloride ions and by dissociating them, but the arrangement of the ions has changed. Please click here for more than information.
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_%28Physical_and_Theoretical_Chemistry%29/Fundamentals/Chemical_Change_vs._Physical_Change
Posted by: hodgesdarm1977.blogspot.com


0 Response to "Does Color Change Indicate A Chemical Reaction"
Post a Comment